Advancing our clinical pipeline in indications with significant unmet need
For more than 15 years, Novocure has performed research and published multiple peer-reviewed articles with preclinical data in more than 15 different solid tumor types in culture and eight different tumor models in vivo.
Preclinical and clinical data continue to suggest broad applicability of the mechanism of action behind TTFields, and we have developed a pipeline strategy to advance TTFields through phase 2 pilot and phase 3 pivotal trials across a variety of solid tumor cancers.
We currently have two ongoing phase 3 pivotal trials, in brain metastases and non-small cell lung cancer (NSCLC), as well as three ongoing or completed phase 2 pilot trials investigating TTFields in pancreatic cancer, ovarian cancer and mesothelioma.

Broad applicability of the mechanism of action
Roza Shnayderman, Head of Novocure’s Israel Biology Lab, joined Novocure in March 2000. During her seventeen years with Novocure, she and the preclinical team have researched the effects of TTFields on a variety of solid tumor cancer cell lines and laid the foundation for Novocure’s expansion into additional clinical trials.
clinical milestones
and next steps

Novocure trials
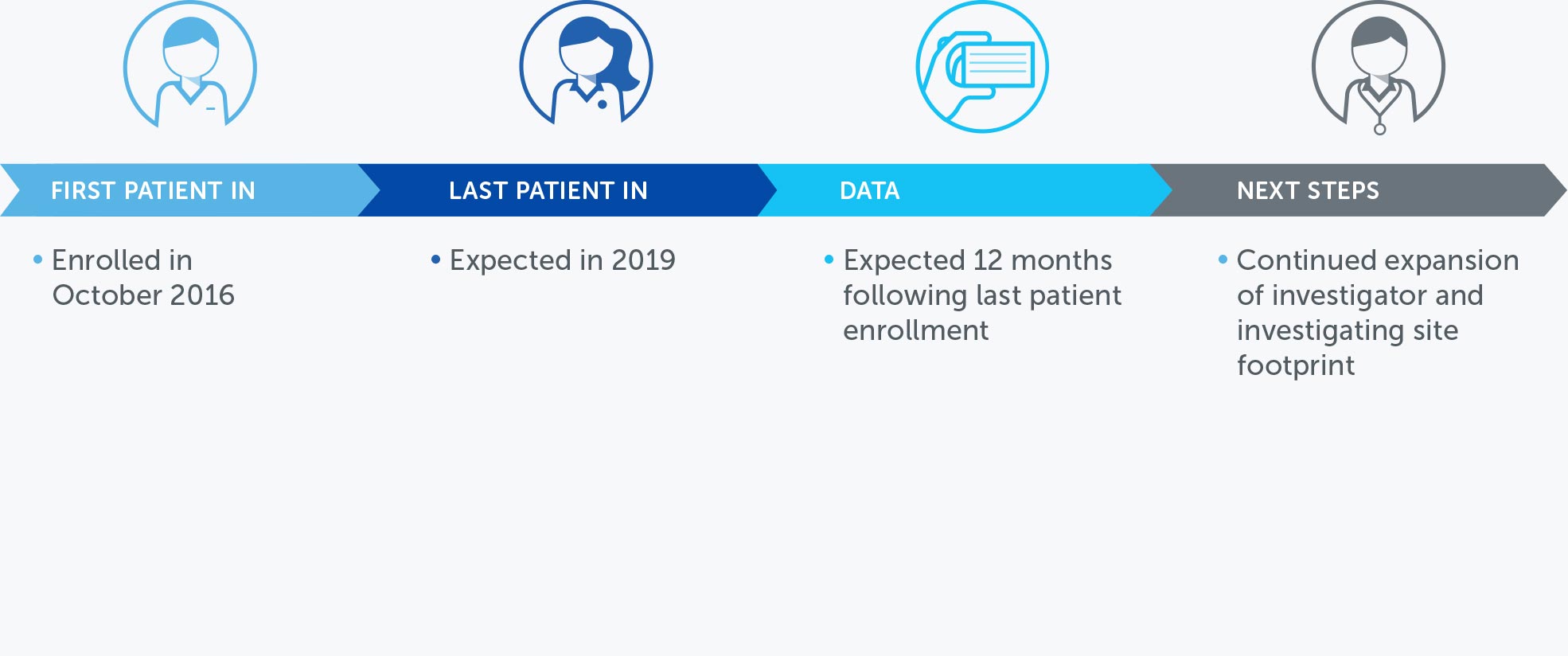
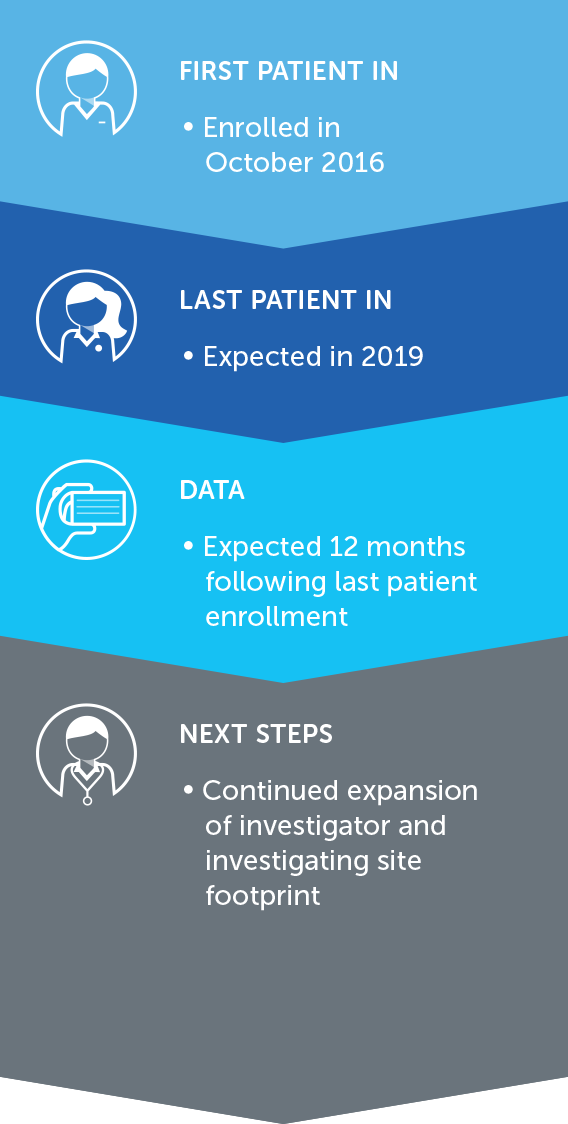
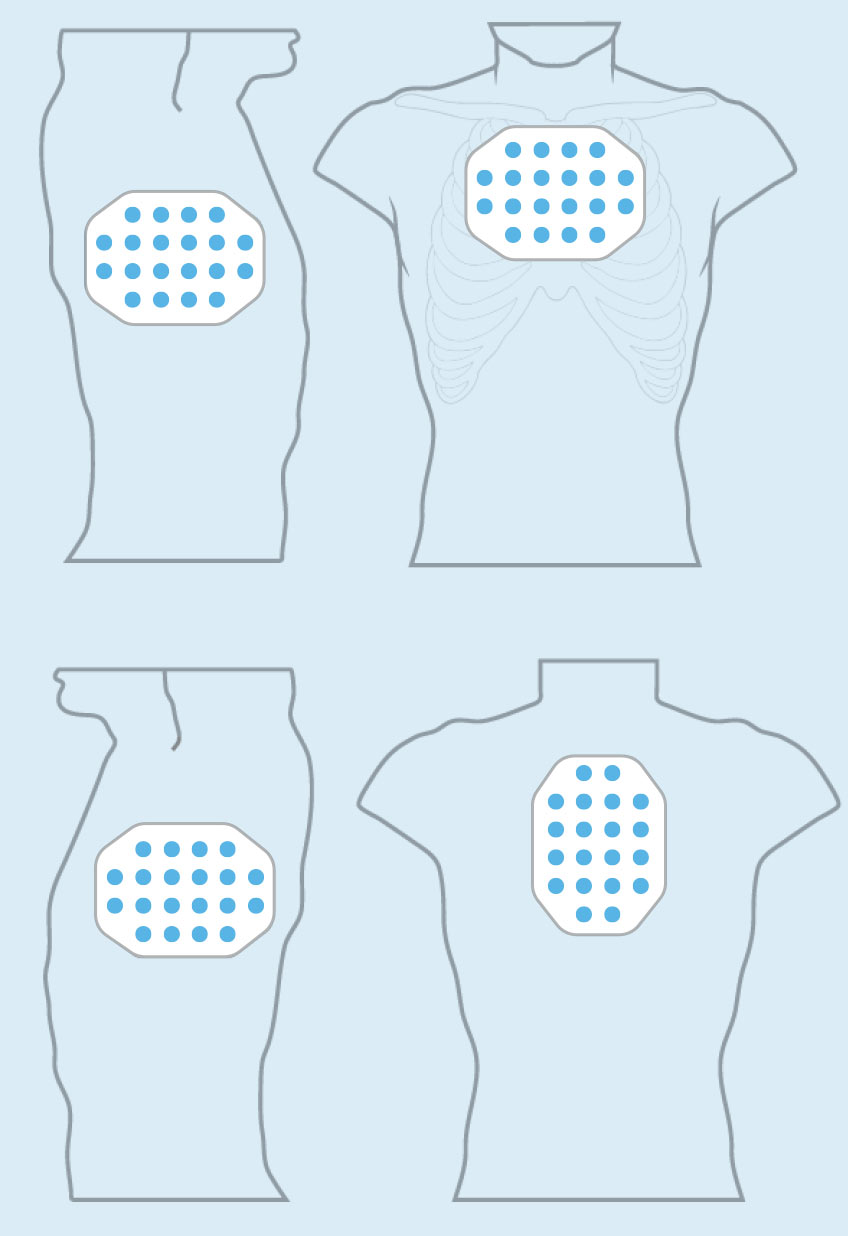
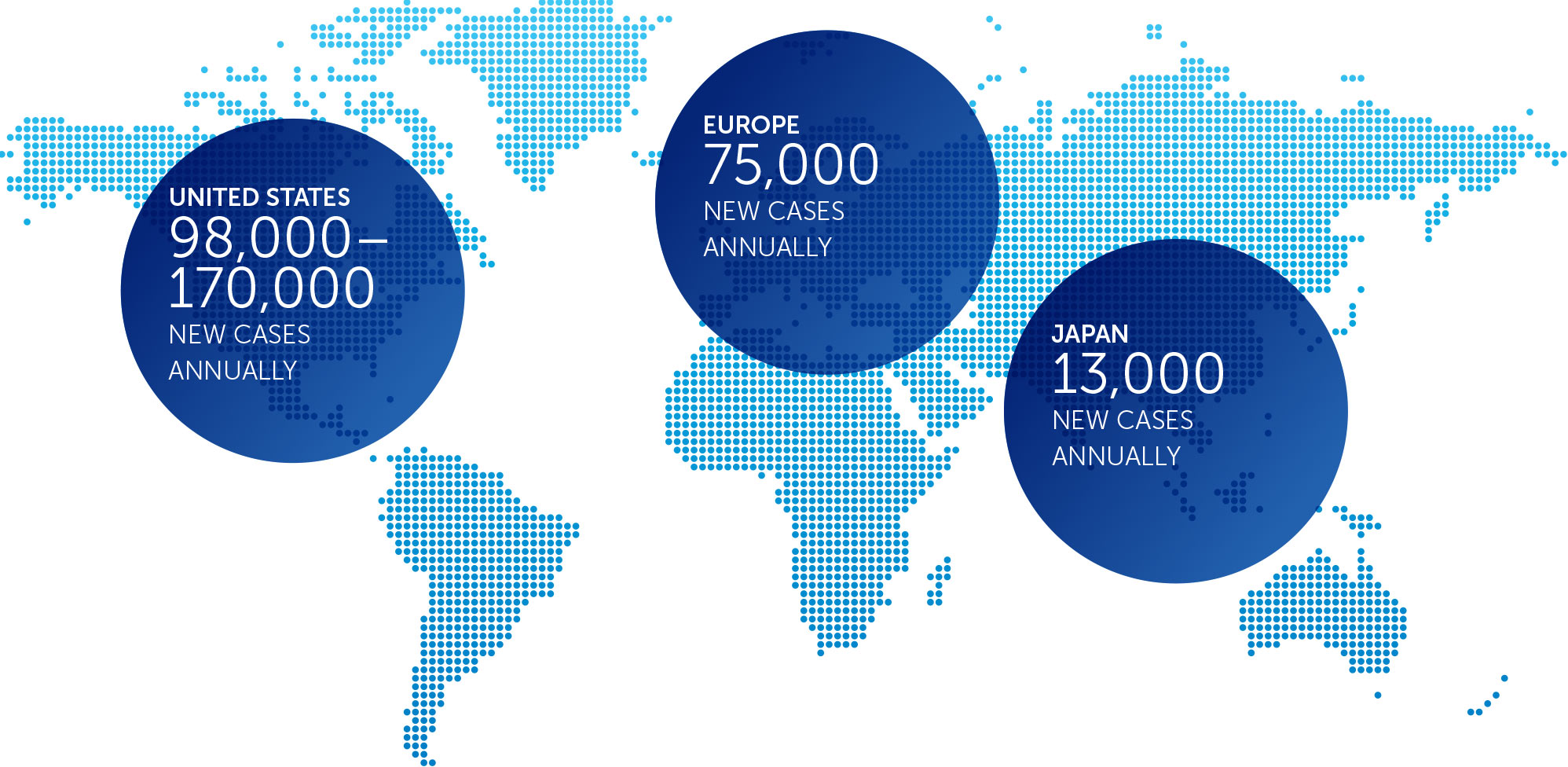
PANCREATIC CANCER
Pancreatic cancer is one of the most lethal forms of cancer globally, killing more than 330,000 individuals worldwide each year. At the end of 2016, we concluded our first phase 2 pilot trial in advanced pancreatic cancer – our PANOVA trial – and data will be presented at the AACR Annual Meeting 2017.
In January 2016, we presented data from an initial 20 patient cohort treated with TTFields plus gemcitabine. These data demonstrated progression free survival and overall survival of patients treated with TTFields combined with gemcitabine were more than double those of gemcitabine-alone historical controls.
In December 2016, we announced topline results from a second 20 patient cohort treated with TTFields plus nab-paclitaxel and gemcitabine. In this second cohort, median progression free survival and one-year survival rate of advanced pancreatic cancer patients treated with TTFields plus nab-paclitaxel and gemcitabine were more than double those of nab-paclitaxel and gemcitabine-treated historical controls.
Based on these results, we plan to open a phase 3 pivotal trial in advanced pancreatic cancer in 2017.
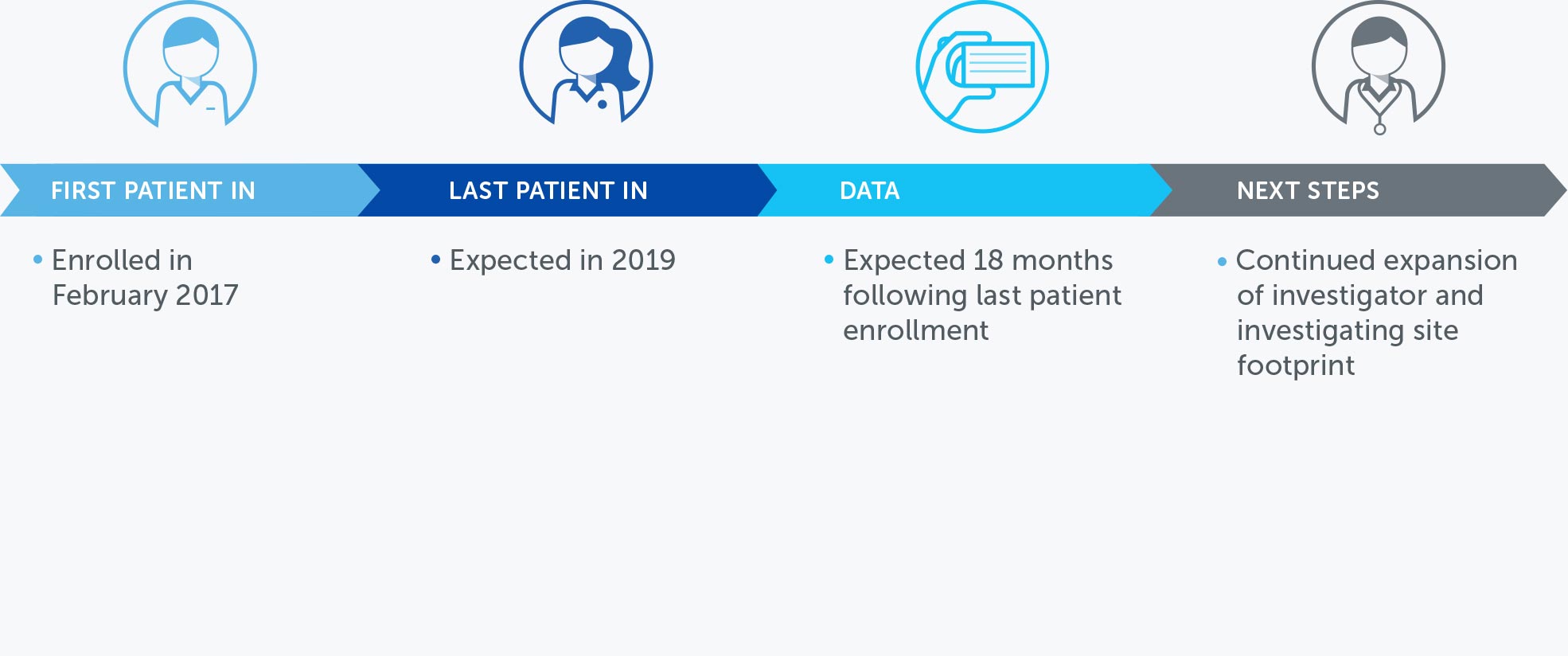
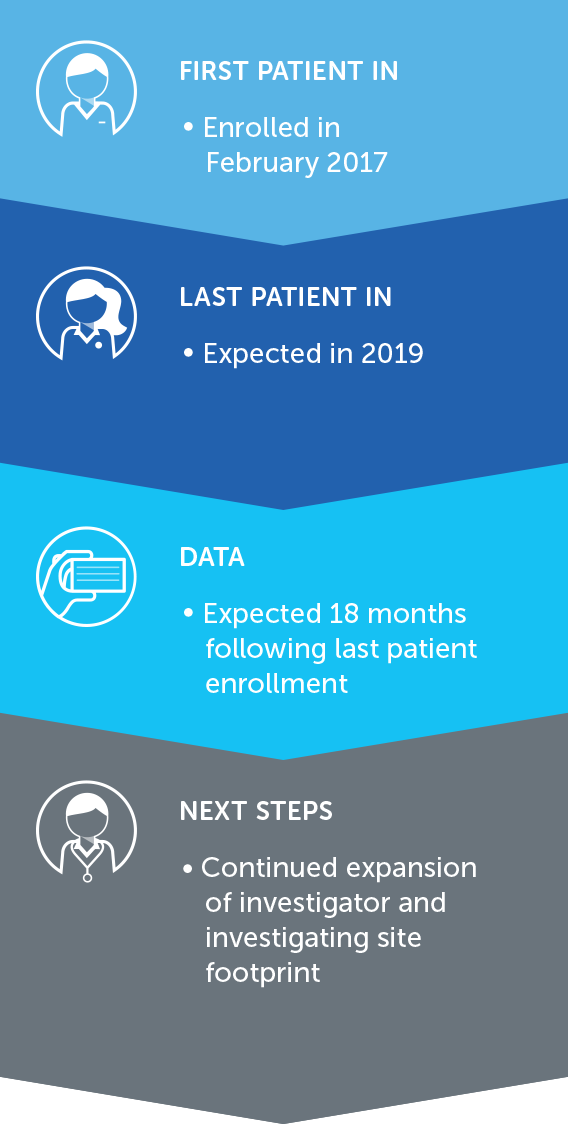
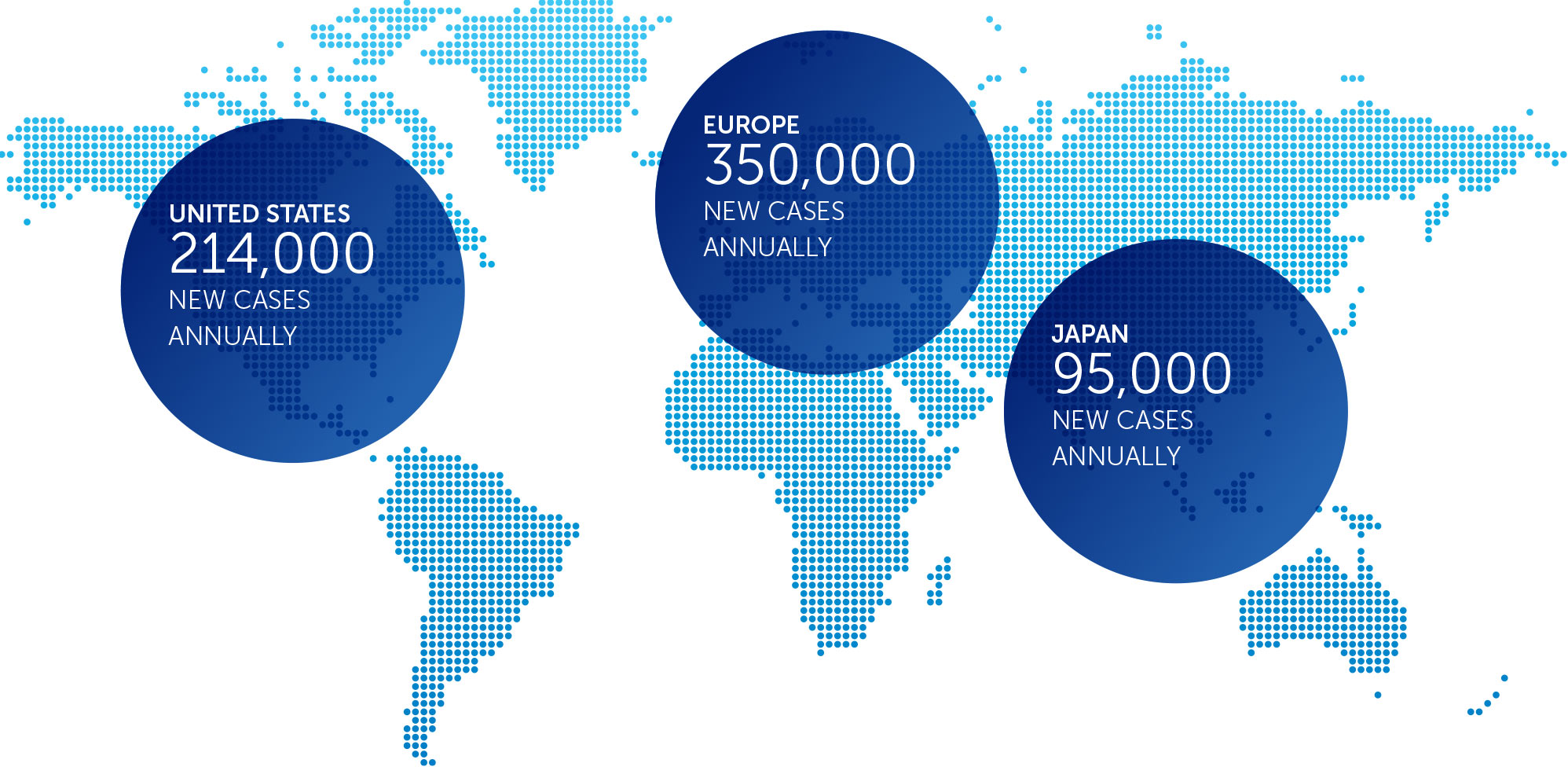
NON-SMALL CELL LUNG CANCER
Lung cancer is the most common cause of cancer-related death worldwide, and NSCLC accounts for approximately 85 percent of all lung cancers. We published data for our first completed phase 2 pilot trial in advanced NSCLC in July 2013.
These results suggested more than doubling of median progression free survival and a 66% improvement in median overall survival in non-small cell lung cancer patients treated with TTFields plus pemetrexed compared to pemetrexed-alone historical controls.
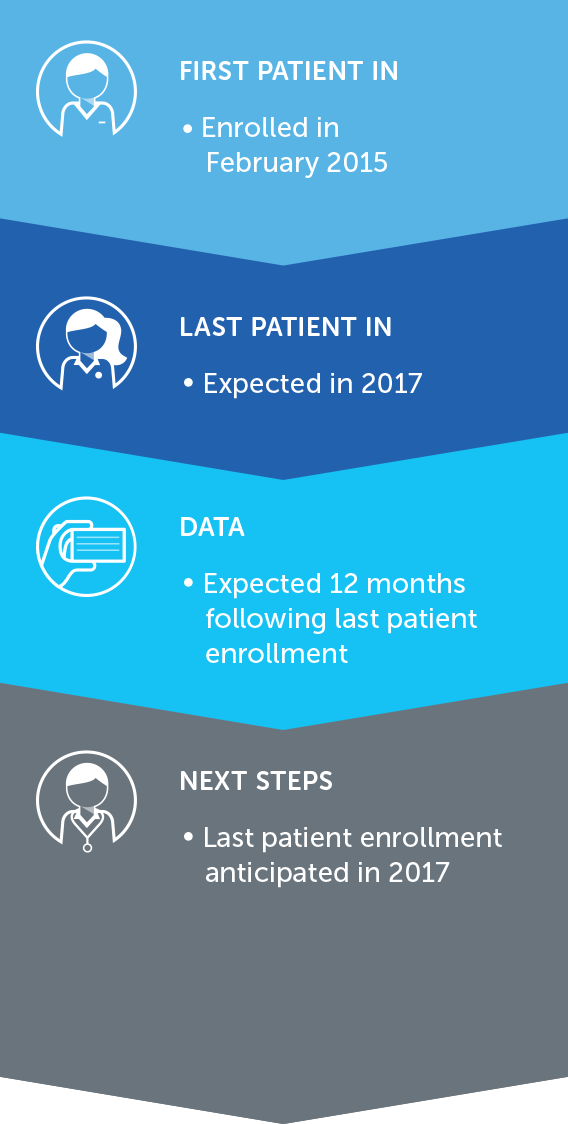
Based on these results, we opened a phase 3 pivotal trial for the second-line treatment of NSCLC – our LUNAR trial – that incorporates the latest standard of care treatment. We enrolled our first patient in February 2017 and anticipate data will be available for presentation approximately 18 months following last patient enrollment.
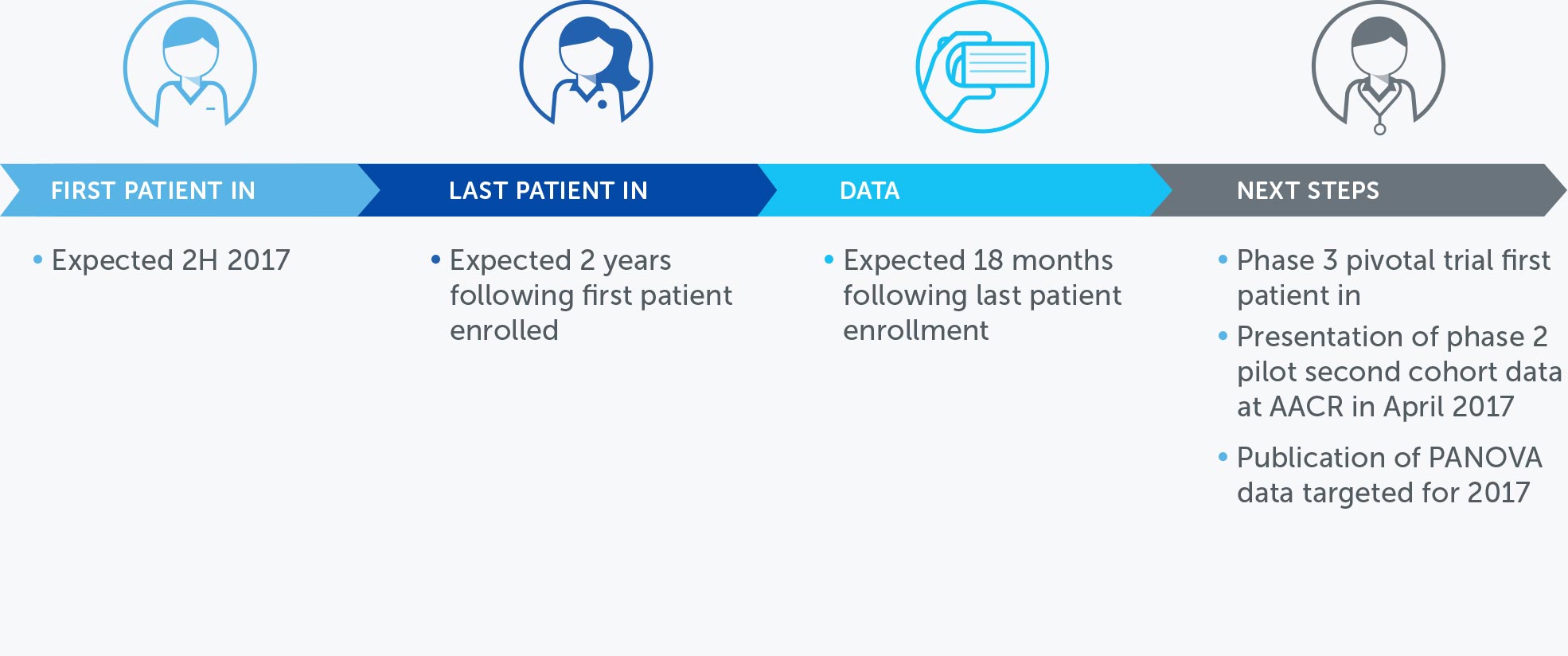
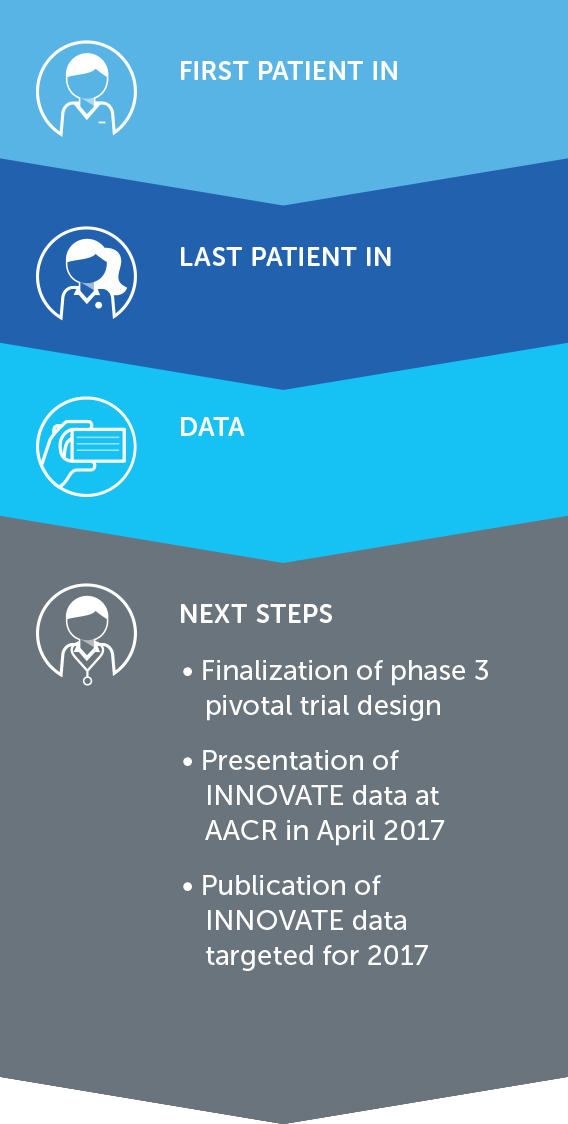
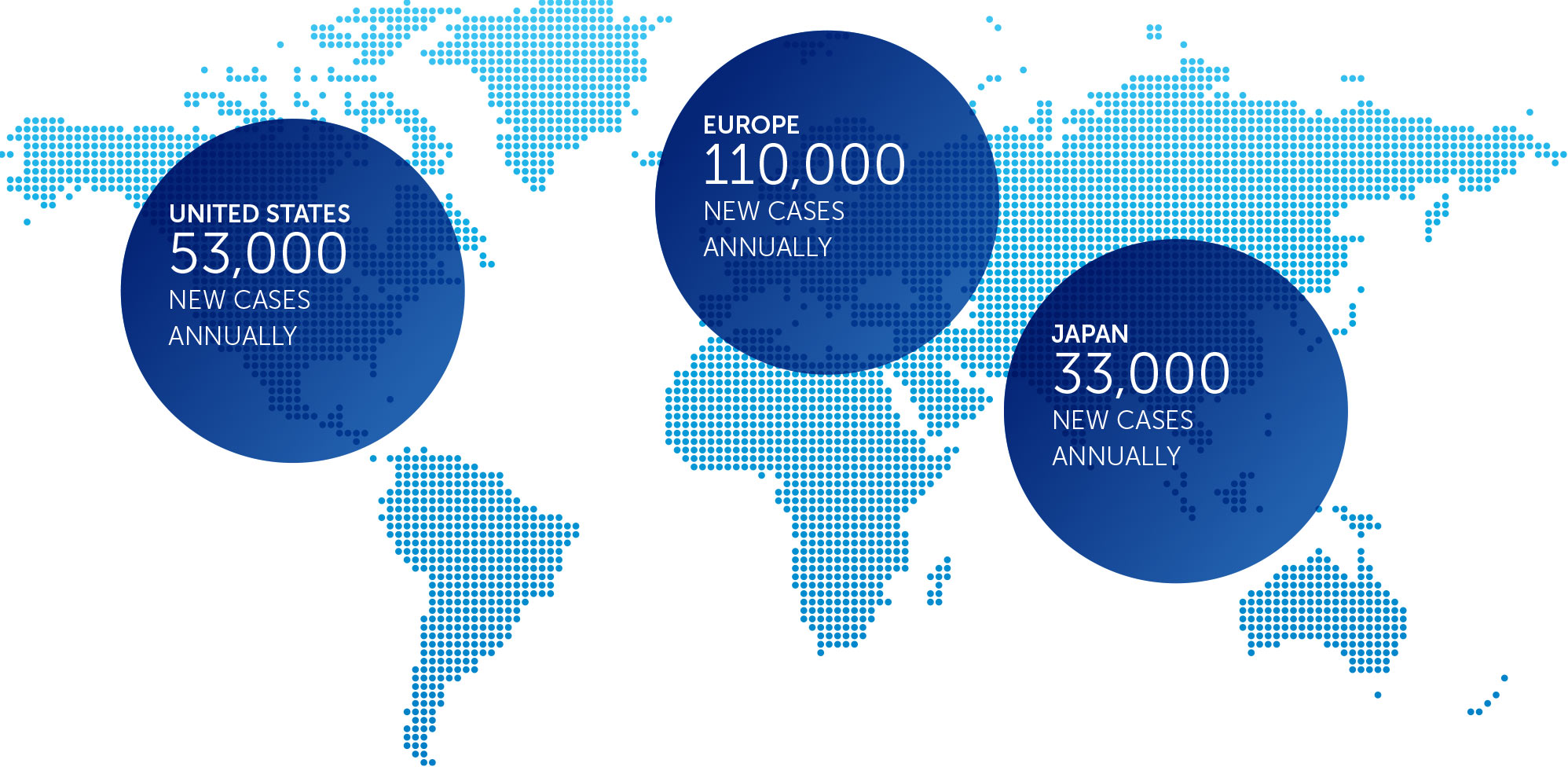
MESOTHELIOMA
Mesothelioma is a rare, solid tumor cancer affecting the lining of the lungs that is strongly linked to asbestos exposure. We currently have an ongoing phase 2 pilot trial in mesothelioma – our STELLAR trial – in which we expect to complete enrollment in 2017.
We presented interim data of the first 42 patients in December 2016. These data suggested that one-year survival rates of patients treated with TTFields combined with pemetrexed and cisplatin or carboplatin were more than 58 percent greater than historical control data of patients treated with pemetrexed and cisplatin alone.
With a minimum of 12 month follow-up after all 80 patients are enrolled, we expect data in 2018.
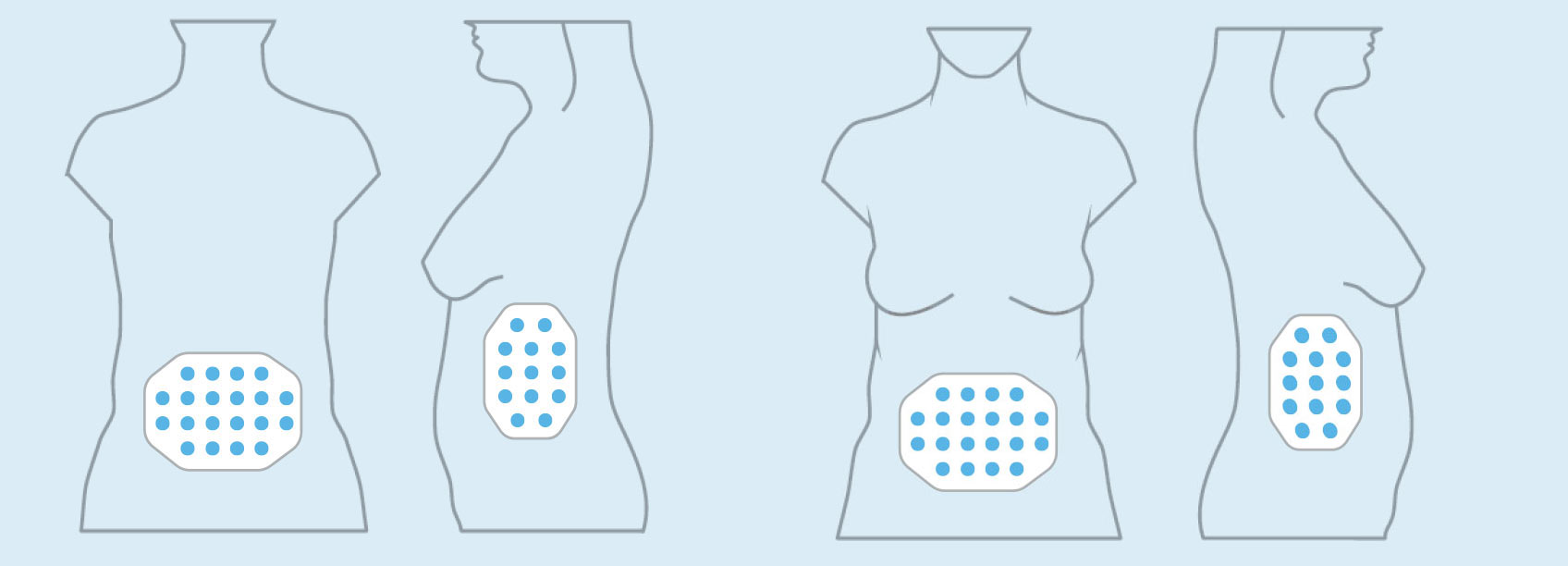
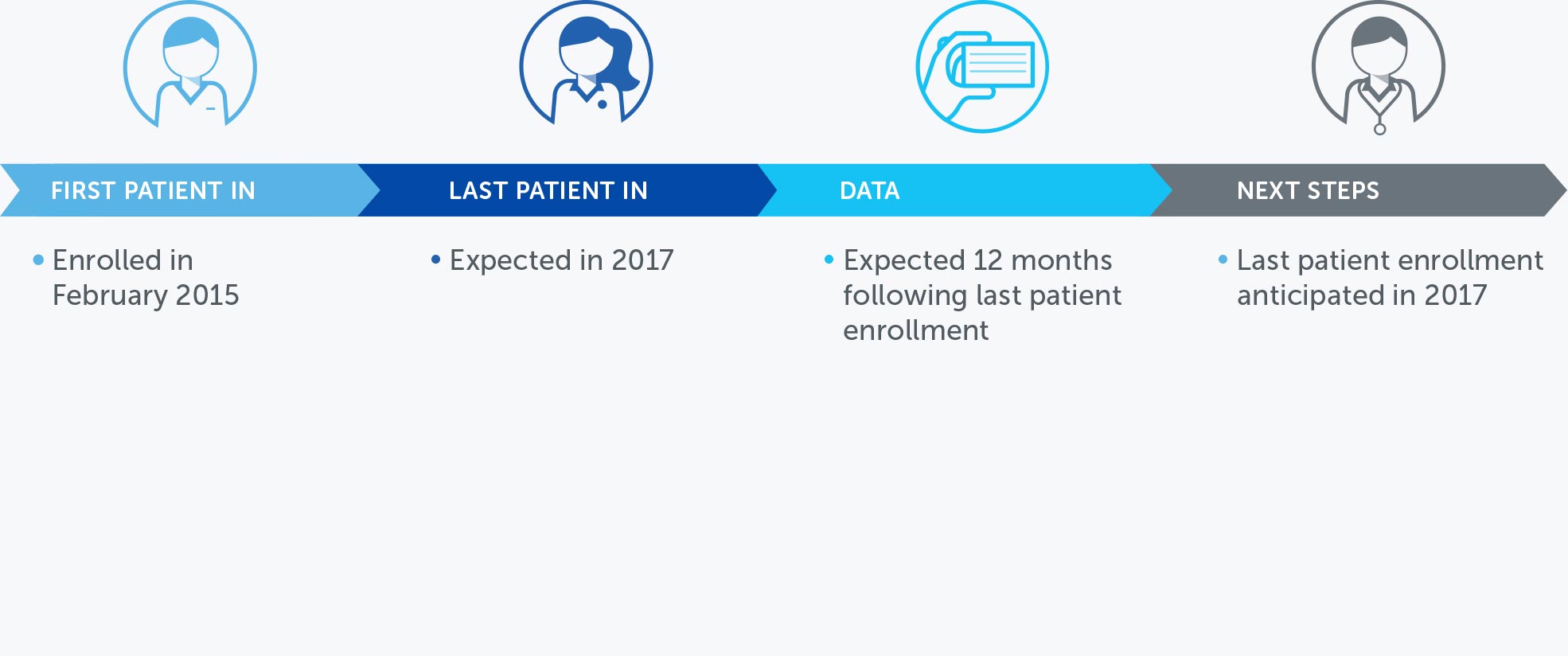
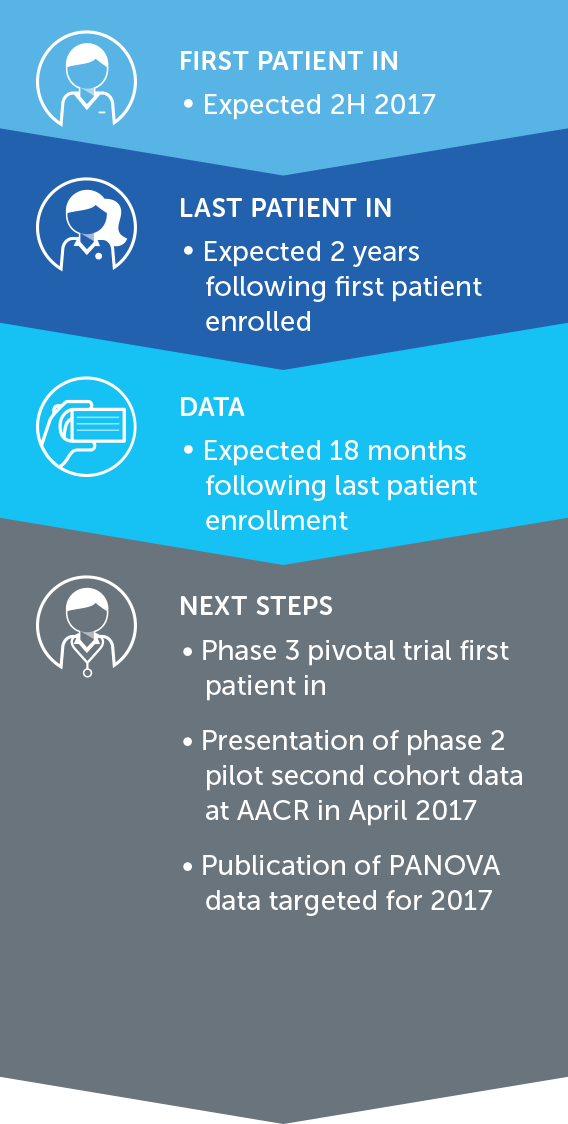
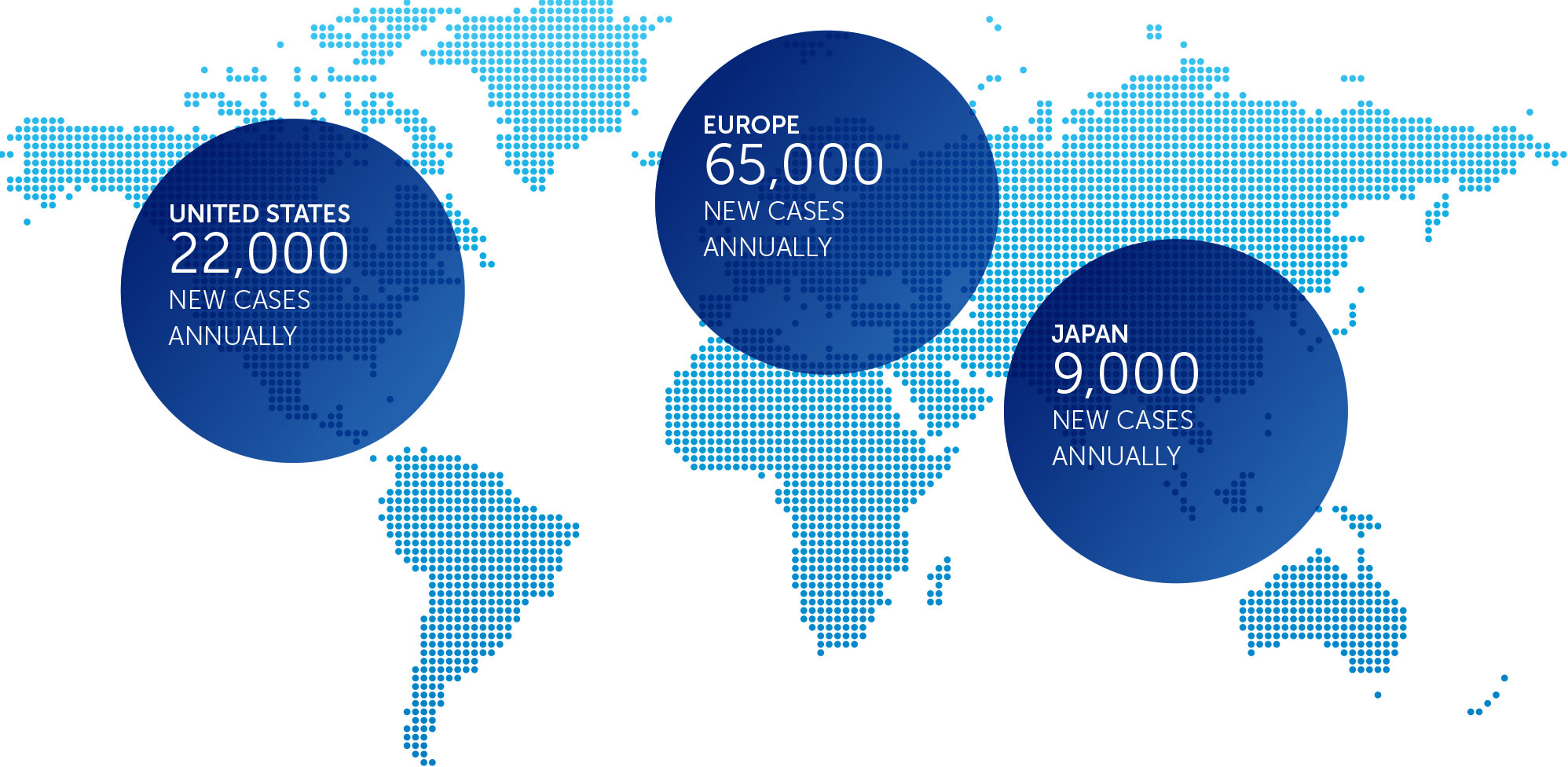
OVARIAN CANCER
In the United States, ovarian cancer accounts for approximately 3 percent of cancers among women, but causes more deaths than any other cancer of the female reproductive system. At the end of 2016, we concluded our first phase 2 pilot trial in recurrent ovarian cancer – our INNOVATE trial – and data will be presented at the AACR Annual Meeting 2017.
We announced topline results from this 30 patient trial at our R&D Day in December 2016. These data suggested a more than doubling of the median progression free survival versus historical controls when treatment with TTFields is added to weekly paclitaxel.
Based on these results, we are developing the trial design for a phase 3 pivotal trial in recurrent ovarian cancer.
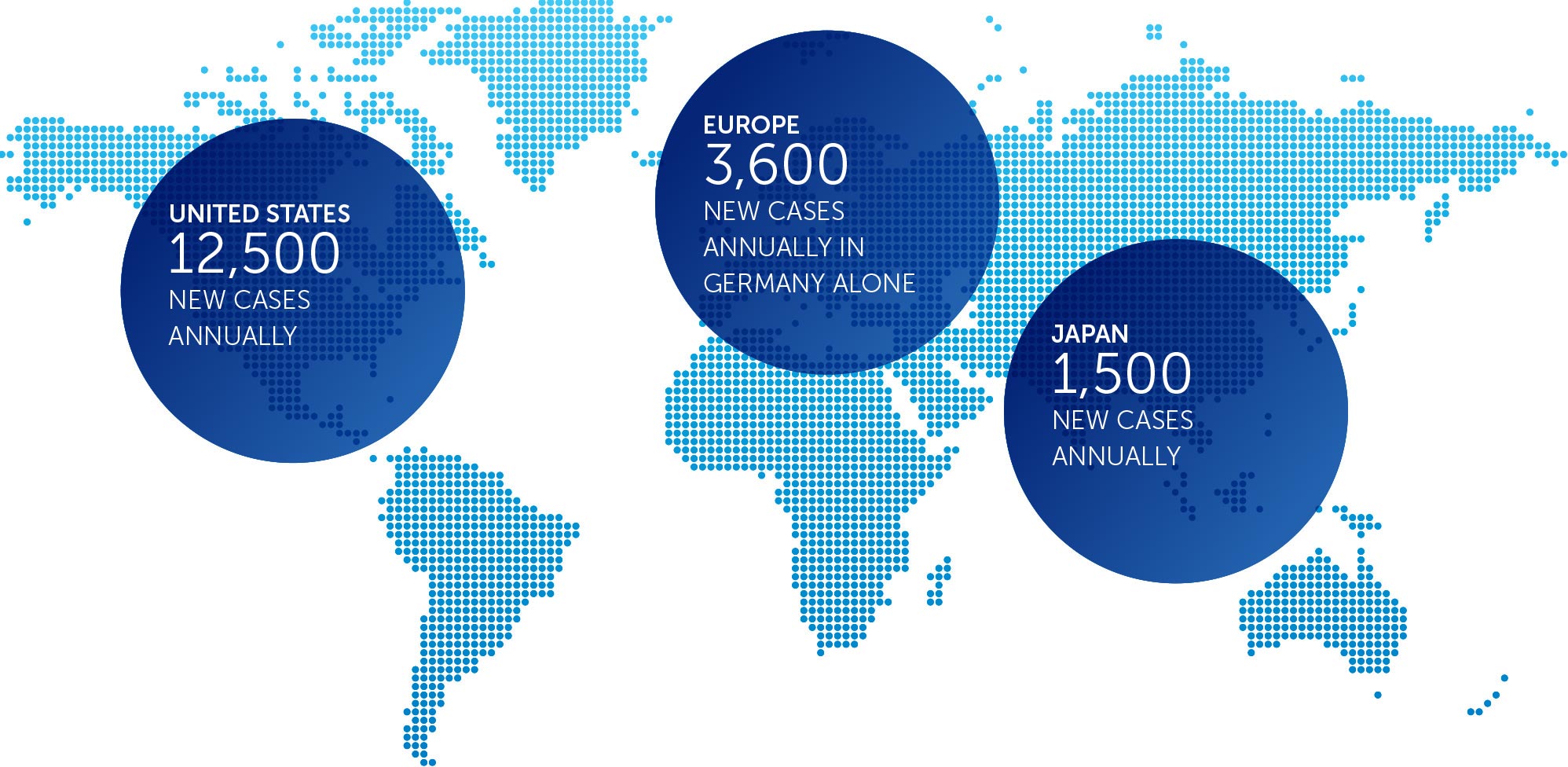
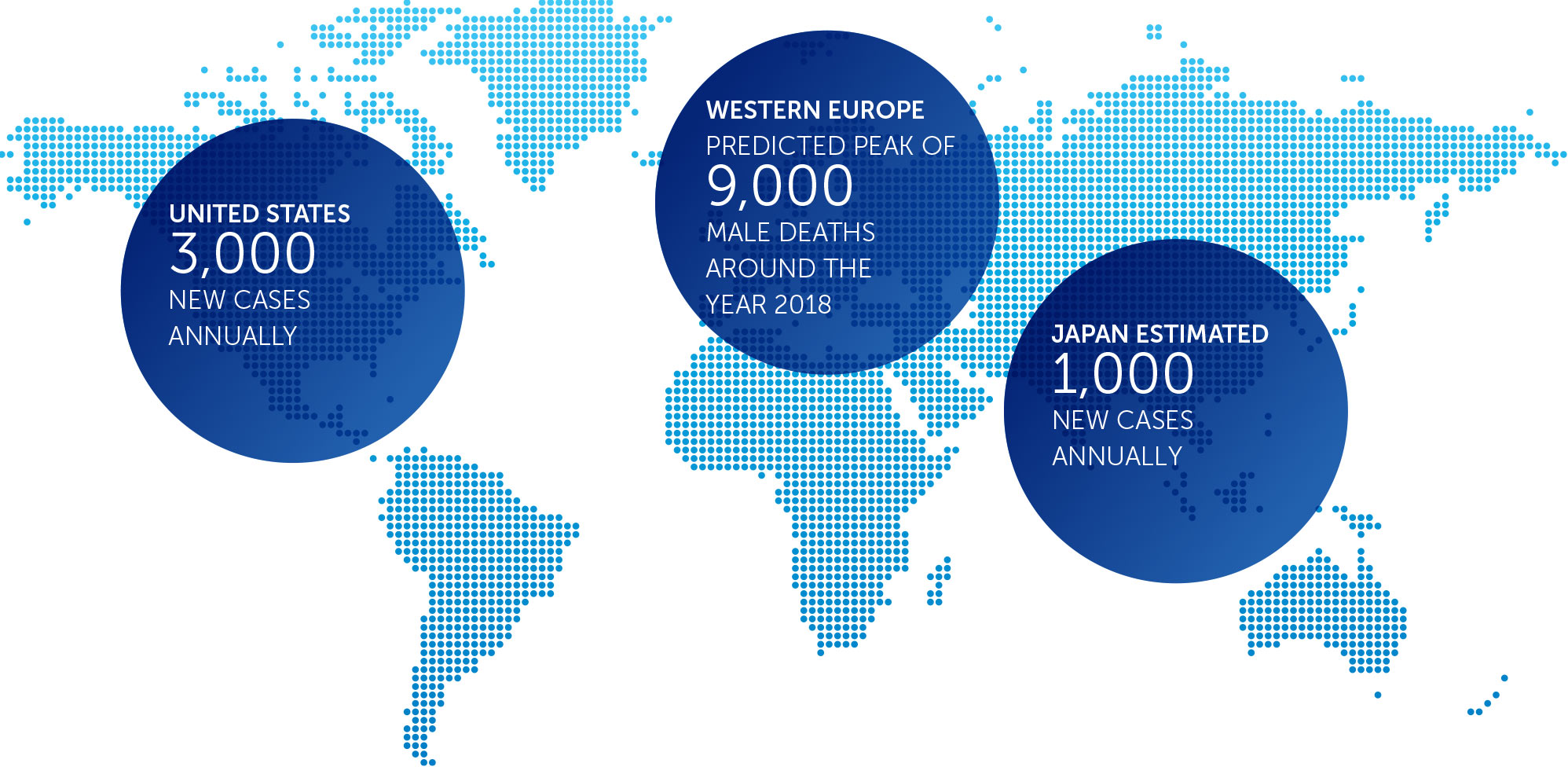
incidence metrics
Novocure engineering
We plan to use the same field generator technology across all indications for which TTFields are approved, but we can specifically target individual solid tumor types by tuning the field generator to the appropriate frequency based upon tumor cell size and adjusting the output power to treat the required tumor tissue volume. As technology for components of our device improves, we have the flexibility to incorporate these advances into our product, subject to applicable regulatory approvals.
Within our GBM indication, our engineering research and development team looks for ways to improve our Optune System by directly incorporating feedback from the patients who receive our treatment. After introducing our lighter, smaller second generation Optune System in mid-2016, we plan to reduce the footprint of our device’s transducer arrays and wires. We hope to launch a less conspicuous, tan colored transducer array in 2017 and we then plan to develop a next-generation transducer array that minimizes the impact of wires to improve overall aesthetics.

Dedication to continual improvement
Victor Kaikov, Electronics Engineer, has been with the company since April 2003. He and the engineering team develop improvements in our treatment systems (subject to applicable regulatory approval), incorporating feedback from our global patient base and advancements in electronics.
Q&A
SHARYN RUPERTI
ASSISTANT CLINICAL TRIAL MANAGER
Sharyn first started working with Novocure about 10 years ago as a Device Support Specialist supporting patients on the company’s EF-11 trial in recurrent GBM. She joined the Clinical Operations Team after receiving her Masters in Clinical Research Administration in 2012.

What does your job entail?
I am responsible for the day to day management of the company’s METIS trial in brain metastases from non-small cell lung cancer. I provide oversight of our clinical research organization and work to ensure study metrics are met. I also visit doctors at participating trial sites and train them on the science of alternating electric field therapy and how to explain the therapy to potential study participants.
How has Novocure changed since your early days with the company?
Novocure has grown significantly in the past several years. When I first started, I was one of six Device Support Specialists hired in the U.S. to support clinical patients in the company’s first phase 3 pivotal study. We are now a global company with more than 450 employees, two FDA approvals and a robust clinical pipeline.
As we continue to develop our clinical pipeline, we are also making connections with and educating doctors interested in our clinical trials from areas of oncology outside of GBM. Today, along with our FDA approvals, we have data published in peer-reviewed medical journals to present to physicians when we introduce our therapy.
There is more excitement today about alternating electric field therapy. There are many doctors who have been waiting to clinically study our therapy for other indications that are difficult to treat.
We also have observed an increased interest from investigators who want to be a part of our studies.
In what ways is Novocure’s approach to cancer treatment different?
I’ll tell you what many doctors tell us. We care. We go out of our way to help patients, and we are closely connected with our doctors. They know that if they have a question, we will respond quickly. I know that every patient matters. Patients on our therapy are not viewed only as a “subject” or study ID number—they are people with loved ones and the company works hard to help each individual patient.

Developing our clinical pipeline
With two FDA-approved indications, three ongoing or completed phase 2 pilot trials and two phase 3 pivotal trial under way, Sharyn Ruperti, Assistant Clinical Trial Manager, said it’s an exciting time to be a part of Novocure.
A patient-forward mission
Assistant Clinical Trial Manager Sharyn Ruperti said she enjoys making a difference. “I feel like I am truly making a difference for patients and their families by providing physicians an additional possible tool to use in their battle against cancer.”